The Twin Threats of PFAS and Microplastics
Welcome to the second issue ContamiNation. Subscribe for free to receive it monthly, and please share this newsletter with others.

The focus of ContamiNation—per-and poly-fluoroalkyl substances (PFAS) and microplastics—might seem like an odd pairing, but they share striking commonalities.
Both PFAS and plastics are human fabrications with no counterparts in the long evolution of life on Earth. Few bacteria or microbes can digest them, although research is underway to identify possibilities.
These invisible contaminants offer no sensory warnings—like taste or odor—to ward creatures away from their potential harm. Even if they did, both threats are now inescapable, present not only in waste streams but in air, rain, drinking water, soil and food.
Plastics break apart, rather than breaking down, fracturing into ever smaller fragments that can cycle through ecosystems for centuries.
The carbon-fluorine bond that characterizes PFAS is the strongest one known in organic chemistry. And a range of chemical precursors morph into more stable, enduring forms of PFAS that can last on a geologic timescale (hence the ‘forever chemicals’ moniker).

Derived from fossil fuels, plastics and PFAS are petrochemical products from an industry that represents the largest industrial energy consumer and the third largest generator of CO2. PFAS manufacturing can produce high volumes of potent greenhouse gases. Plastic production often incorporates and generates PFAS, which are also used in extraction activities like oil drilling and methane gas fracking.
Chemical manufacturers such as 3M and DuPont, taking cues from fossil fuel corporations, have spent decades withholding evidence of the harm their products inflict.
The federal government characterizes PFAS and microplastics as “contaminants of emerging concern,” but the term seems disingenuous: The U.S. Environmental Protection Agency (EPA) has known about PFAS threats since 1998 and once acknowledged that “microplastics have been identified as a potential environmental concern since the 1970s.”
What’s “emerging” is not the contaminants themselves but the realization that we face a significant threat to public health and ecological well-being from toxic substances that remain largely unregulated.
PFAS and microplastics build up in plant and animal tissue, and become more concentrated moving up the food web. Both contaminants are widespread in human blood, placentas and breast milk. There’s a long and growing list of health concerns associated with PFAS (see figure), and microplastics appear to cause metabolic disruptions, accumulate in organs and damage cells.
What’s “emerging” is not the contaminants themselves but the realization that we face a significant threat to public health and ecological well-being from toxic substances that remain largely unregulated.
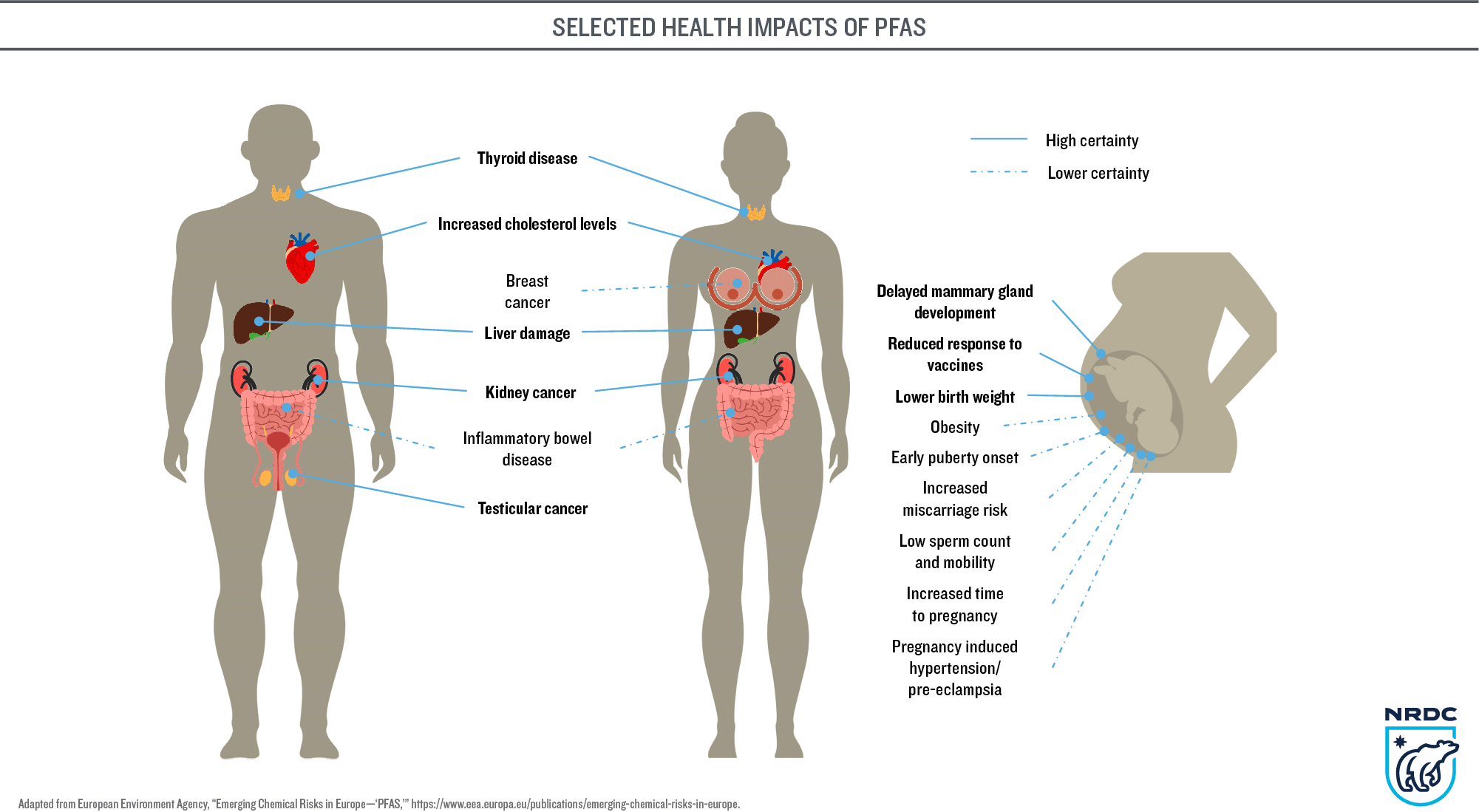
More disconcerting still, these two threats don’t act in isolation. Microplastics can serve as a vector, potentially carrying an added load of PFAS (and other toxic chemicals) that have adsorbed to their surface. This transport has been noted in earthworms and fish, where researchers observed that PFAS may “enhance the permeability” of microplastics, allowing them to pass more readily through the small intestine and into the circulatory system (and hence into organs and tissues).
A team of researchers led by the University of Maine is working to develop a model that could help predict the bonding dynamics between PFAS and microplastics in fresh water and saltwater based on factors such as the type, size, shape and charge of the microplastics, the type of PFAS, and the water chemistry (such as salinity and pH level).
In ways we’re just starting to grasp, PFAS and microplastics are literally conjoined. What that fusion means for our health and our world remains to be seen.
Research: Finding Fluorinated Chemicals in Consumer Goods
An academic physicist might seem like an unlikely candidate to feature in consumer health stories, but the PFAS research of Graham Peaslee, a professor at Notre Dame University, routinely generates media attention. His lab’s findings have prompted many corporations to reformulate products.
Peaslee developed a rapid-screening technique known as PIGE (“piggy”), short for proton-induced gamma emission, in which gamma rays powered by a particle accelerator reveal total fluorine content. That acts an effective proxy for PFAS because there are few other sources of fluorine in products.
PIGE reveals the breadth of PFAS present, whereas conventional lab methods typically assess fewer than 100 PFAS compounds of the nearly 15,000 PFAS identified to date.
Peaslee’s lab has tested a wide range of products—from cosmetics and firefighter turnout gear to fast-food packaging and school uniforms. His students handle about 5,000 samples a year, but far more materials could be tested if this approach were replicated outside an academic lab.
Through a contract with the Department of Defense, Peaslee and fellow researchers are now at work on a portable “PIGE” prototype (small enough to fit in a van) that could run 100,000 samples a year with just one technician needed. The testing unit might cost $1 million, but the large sampling volume could be a “game-changer” for expediting water sampling in the field, he says.
Science pushing policy
One product Peaslee’s lab tested earlier this year is high-density polyethylene (#2) plastic containers that were fluorinated, a process that inadvertently creates PFAS that can leach into the contents (such as pesticides, personal care products, household cleaners or food flavorings).
This new source of exposure was discovered when Kyla Bennett, director of science policy for Public Employees for Environmental Responsibility, suspected that PFAS contamination in her community’s water supply came from pesticides aerially applied in the vicinity. The PFAS those pesticides contained, the EPA confirmed, came from the storage containers.
“Nobody saw this coming,” Pam Bryer, a toxicologist with Maine’s Bureau of Pesticide Control said earlier this month. By one industry estimate, 20 to 30 percent of the plastic containers used for pesticides and fertilizers may be fluorinated, according to Bryer’s research. (Two scientists wrote about this risk more than a decade ago, but that study unfortunately garnered scant public attention.)
In early December, the EPA ordered the primary manufacturer of those containers, Inhance Technologies LLC, to cease their production by March 2024. The action came more than three years after Bennett notified the EPA of the risk: “It’s a relief that EPA is finally taking action to halt this dangerous practice,” she observed.

News of Note
The latest rounds of negotiations on an international plastics treaty ended with little progress, the Associated Press, Inside Climate News and Nature report. Despite efforts to strengthen conflict-of-interest policies, the Center for International Environmental Law calculated that there were 143 lobbyists present from the fossil fuel and chemical industries, a pattern increasingly evident in U.N. climate talks as well.
At those treaty negotiations and elsewhere, oil companies and oil-producing nations often promote plastic recycling as a solution to the escalating plastic waste problem (which generates the microplastics we’re inhaling and ingesting). But a new study reported by Environmental Health News finds that recycled plastics contain even more chemicals and higher levels than new plastic materials. In 2021, only 5 percent of U.S. plastics were recycled.
An article in Civil Eats highlights the growing threat of microplastics in soils and foods due to heavy use of plastic in agriculture. (And where there are microplastics, there may be PFAS).
Shortly after the public release of a letter that United Nations human rights investigators wrote outlining their concerns to Chemours (a chemical manufacturer spun off from DuPont) and others, the EPA withdrew its conditional approval for Chemours to import waste containing PFAS from its plant in the Netherlands to its Fayetteville, North Carolina facility (renowned for its pollution of the Cape Fear River watershed). The company had erred in reporting the import volume, EPA noted, but the agency’s concerns included the facility’s historic releases. The U.N. investigation was prompted by the grassroots group Clean Cape Fear, working in collaboration with the U.C. Berkeley Environmental Law Clinic.
A poll conducted by Texas A&M found that most Americans do not know what PFAS are or have any sense of their potential risks. More than 97 percent did not believe their drinking water was affected (despite a U.S. Geological Survey study released in July estimating that at least 45 percent of the nation’s tap water contains one or more PFAS). Nearly half of participants had never heard of PFAS and 31 percent had heard the term but did not know what PFAS are. The survey found no statistical differences reflecting differences in race, gender or age.
Good Resources
To learn how to avoid PFAS in some household goods, review the product research commissioned by consumer advocate Leah Segedie, in collaboration with Environmental Health News and the Institute for Green Science at Carnegie Mellon University. They have tested for total organic fluorine (a marker for the full range of PFAS present) in toilet paper, butter wrappers, dental floss, cooking oils, nut butters and more. Their EPA-certified lab uses a different method than the PIGE one described above (instead using oxygen flask combustion and ion-selective electrode).
On the consumer goods theme, here are three discussions worth listening to:
Linda Birnbaum, former director of the National Institute of Environmental Health Sciences, and consumer advocate Leah Segedie discuss PFAS testing of consumer goods with Brian Bienkowski of Environmental Health News.
Sian Sutherland, who works with the business community to reduce reliance on plastics, talks with Nate Hagens on The Great Simplification podcast; and
Alden Wicker, author of To Dye For (a new book on toxics in the fashion industry), is interviewed by Tanya Mosley on Fresh Air.

Good Riddance
To the extent possible, avoid takeout food packaging due to potential PFAS and don’t compost even “compostable” containers. Graham Peaslee and fellow researchers sounded the alarm about fast-food wrappers and containers five years ago, prompting some companies to move toward PFAS-free packaging. But a 2022 study done by Consumer Reports found some fluorine still in the replacement products, although at lower levels. As toxicologist Jamie DeWitt, of East Carolina University, observed to Consumer Reports, this is ultimately a collective and regulatory problem: “When faced with chemicals that are as ubiquitous as PFAS, it’s a little unfair to ask individuals to limit their exposure. These chemicals are made by corporations, and the onus shouldn’t be on the individual.”


In terms of response and policy, the toxicologist you quoted at the end hits the nail on the head: “These chemicals are made by corporations, and the onus shouldn’t be on the individual.”